Fluorescence microscopy of fungi in native soil – Improvement by additional substances.
Christian Jensen and G. Lysek
Institute of Systematic Botany and Plant Geography, Free University Berlin; Altensteinstr. 6, D-14195 Berlin.
First published by Microscopy and Anylysis in September 1995 Issue No: 49 Pages: 7 – 9
Introduction
The staining by fluorescein, applied in the form of fluorescein di-acetate, FDA, (SÖDERSTRÖM 1977; BAATH & SÖDERSTRÖM 1988), has allowed the microscopic observation of predacious fungi in their native soil. This was possible by using the „hanging drop,‘ technique and after developing a procedure for cutting the soil (JENSEN & LYSEK 1991; JENSEN 1994). The application of these methods allowed also the routine observation of these fungi during longer and representative periods (SAXENA & LYSEK 1993); it also provided proofs that growth, development and predacious activities virtually resembled those known from agar-cultures. In spite of this success, the method was not completely satisfying. The main disadvantage resulted from the FDA, which is not fluorescing by itself, but only after uptake into living organisms and subsequent liberation of the fluorescein by hydrolytic cleavage (SÖDERSTRÖM 1977). As a consequence, only those (parts of) living cells or hyphae show the fluorescence, which are actively metabolizing and thus have the hydrolytic capacity to transform the FDA (CORREA et al. 1986). Other parts, especially older but still vital or dormant organs or structures, are not stained and hence not visualized by this otherwise convenient method. Another problem hampering the broad usage of this method is the fast photo-fading of the fluorescein, especially at higher magnifications. It limited the time available for observations and interfered heavily with the photographic documentation. Experiments thus were started, to use additional staining and reacting substances. Among a number of chemicals few turned out to be valuable, but with their help the method could be improved considerably.
Material and Methods
Preparation of the soil:
The soil samples were prepared according to JENSEN & LYSEK (1991): the soil samples were moistened (if necessary) to enhance the adhesive contact of the soil, which allowed putting it on a cover slide and turning it upside down to obtain a hanging drop. To keep the soil in its native structure, further manipulations were strictly avoided.
Fluorochroming:
The fluorescein di-acetate (FDA) was obtained from Sigma (F-7378). A stock solution (0.05 Z in acetone) was kept dark and cool; from it the working solution was prepared by adding 100 µl to 5 ml buffer solution. This working solution could be used for 20 min at the most, since it degraded rapidly. For details see JENSEN (1994) For other fluorochromes see results.
Microscopic equipment:
Microscope A Leitz Dialux 20 was used for these investigations, completed with a Pleomopak 2.4 fluorescence unit equipped with a mercury arc lamp HBO 50 W (Osram). Filter units: The filter units A, E3, 13 and N2 were used to get an optimal fluorescence from the various fluorochromes. Photographic equipment: The Dialux 20 microscope was additionally equipped with a Wild photo-tube and camera MPS 11; connected to a Wild MPS 15 semiphotomat for semi-automatic regulation of the exposures. Several normal speed Kodak Ektachrome 100 and Ilford Delta 400 & 100 negative and slide films were used.
Results: 1. Fluorochromes
Calcofluor white M2 R new:
This well-known fluorochrome is widely used as and optical brightener. It stains 1,4-linked polymers (cellulose, chitin) and thus hyphal and cell walls (SENGBUSCH 1983; DOLAN & McNICOL 1986). GULL & TRINCI (1974) used it to stain the walls in the apical region of growing hyphae; but old structures which are still active but no longer metabolizing intensively are also visible due to the staining of hyphal walls. An additional advantage of calcofluor W is its stability of the fluorescence as well as its binding to the polymers: even permanent mounts exhibited the fluorescence after long times. Fig. 1 shows the fluorescence of calcofluor W in Arthrobotrys oligospora. The substance was obtained from Sigma (F-6259). It was applied by mixing 50 mg calcofluor white to 5 ml buffer solution (Tris-buffer pH 9.00) as a stock solution. The working solution was obtained by dilution 100 µl stock solution to 9.9 ml buffer. This working solution was quite stable.
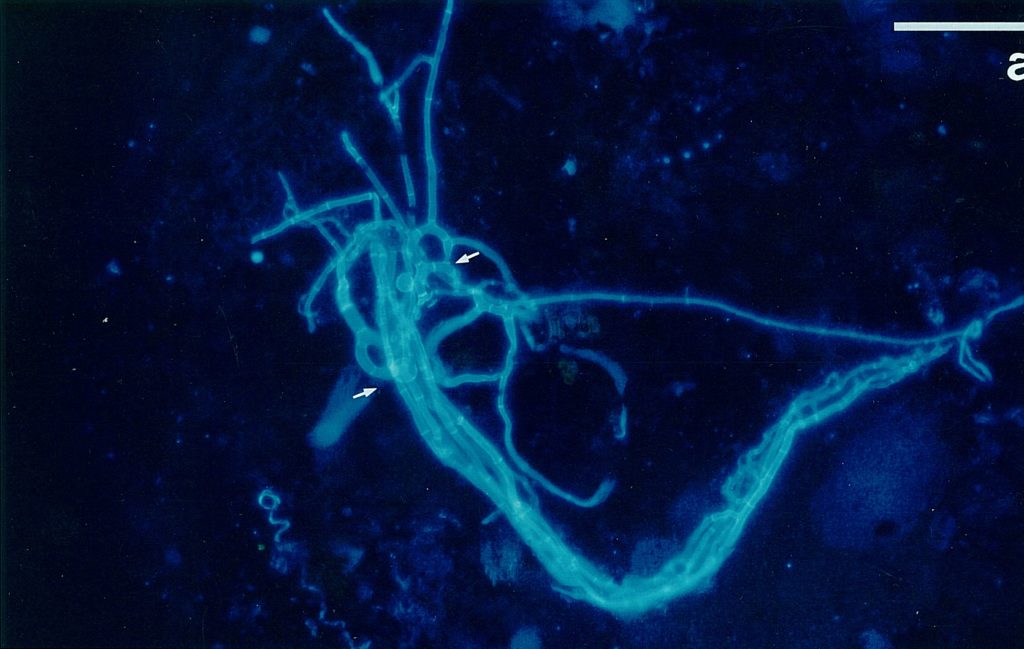
Rhodamin B:
This is a well-known stain for lipid substances (GRIFITH 1984; PEARCE 1984; HEATH 1988). During these experiments it could be used only after fixation by heat or ethanol; the results, however, were not satisfactory. As a vital stain it could be used only in combinations with other fluorochromes as shown above. In these cases it gave better results with assimilative hyphae inside the nematode corpses and allowed differentiation of conidia, which exhibited a yellowish-red fluorescence.
Further fluorochromes:
The following substances were tested, but did not give satisfactory results with predacious fungi. Their application, however, might be useful with other investigations.
Acridinorange: A well-known vital stain, which gave in our studies good results with bacteria only.
DAPI (41-6-diamino-2-phenyl indole): Widely used for fluorochroming of nuclei (RAJU 1982); in soil, however, the results were quite bad.
Ethidium bromide: Without fixation by ethanol bacteria were stained exclusively; in soil it did not yield good results.
Combinations:
To get all fungal – or hyphal – structures stained, FDA fluorochromation was often completed by the addition of calcofluor W. Though the pH optima were in both cases different (pH 6.0 for FDA and 9.0 for calcofluor white), an overlapping region was found at pH 7.0. Since, in addition, the Pleomopak 2.4 equipment allowed the changing of the filter units on a revolver, differentiated observations with both the stains separately could be done simultaneously. Double exposures allowed the documentation of the entire information, as shown in Fig. 2 and 3. In some cases the treatment was completed by addition of rhodamin B, which allowed a further improvement of the picture. Its role in the fluorochroming of living i. e. active fungi is not clear, as this substance demands previous fixation – which was not possible with FDA-staining. Fig. 4 shows the result of such a triple staining with FDA, calcofluor white and rhodamin B.
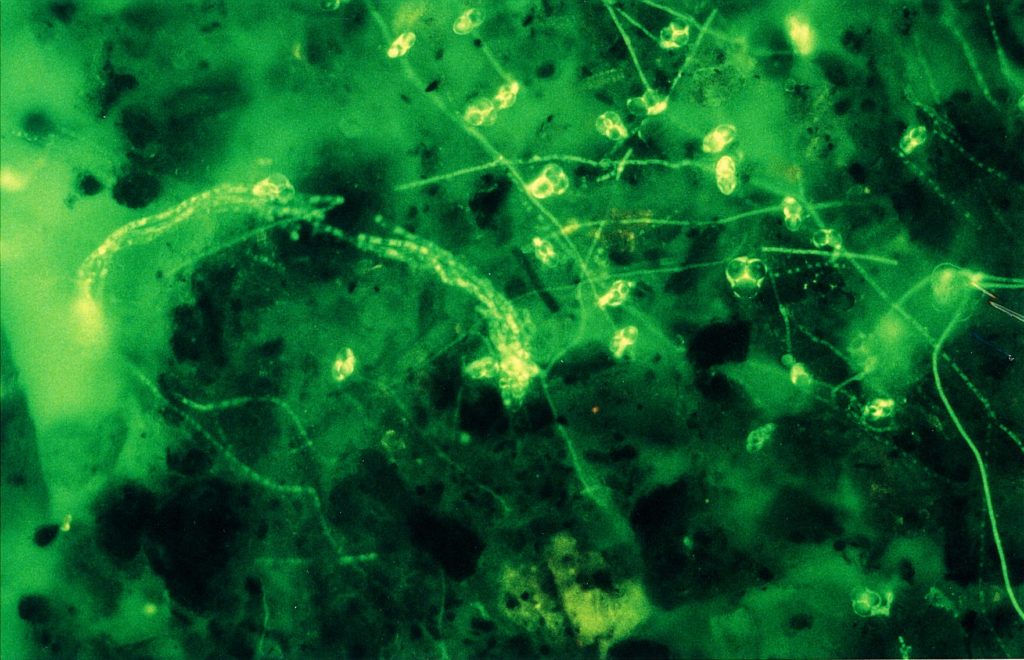
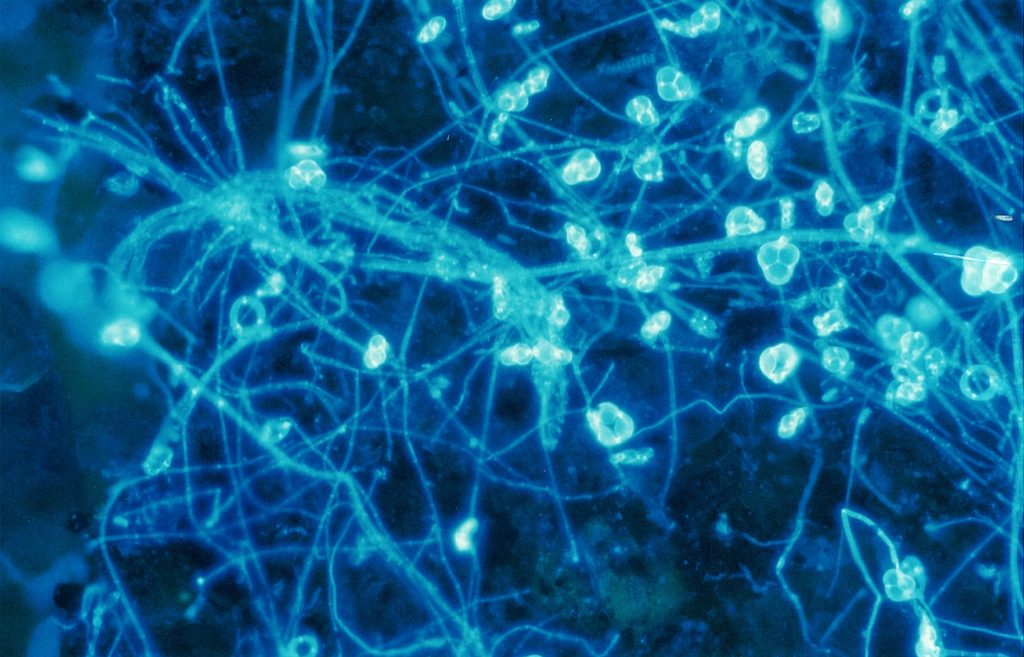
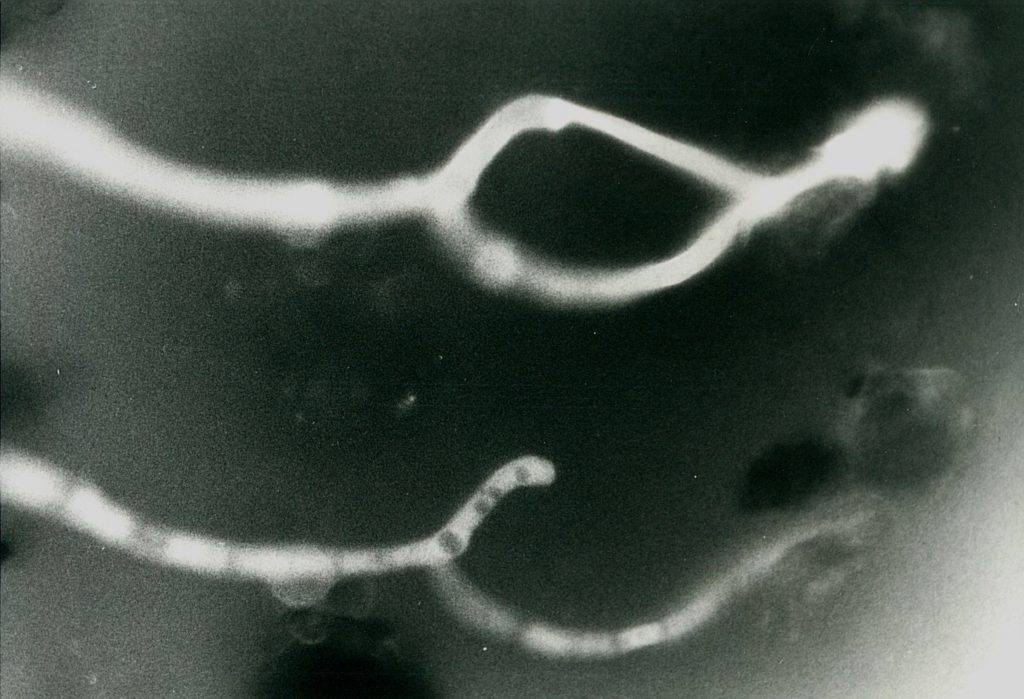
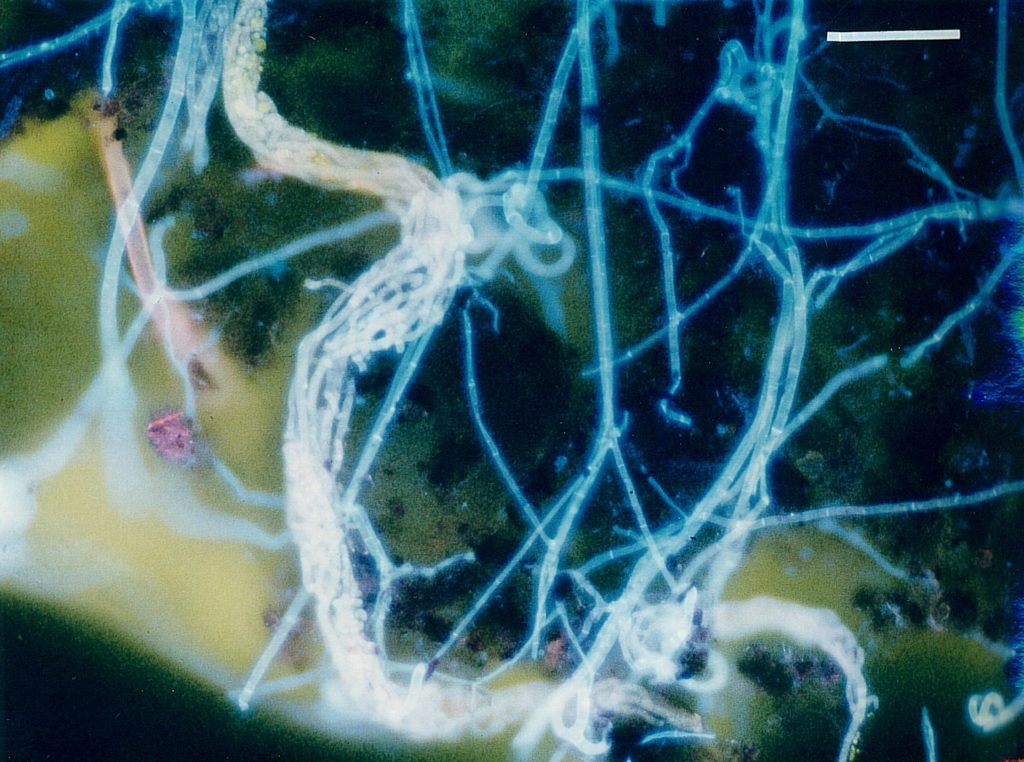
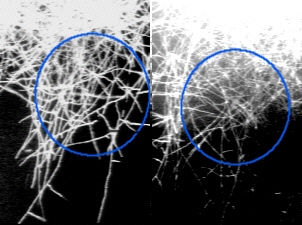
Discussion
With these improvements some major inconveniencies of the FDA-method of fluorescent staining for observation of soil organisms in situ could be overcome in as all fungal structures could be observed – while still the metabolically active parts are stained exclusively by the FDA and thus can be located easily. With this level the technique now allows the routine study of fungi inside the soil (SAXENA & LYSEK 1993; JENSEN 1994). Further – and desirable – advances are aimed to the differentiation between the various fungal structures – e. g. conidia, inactive or resting spores, chlamydospores and sclerotia etc. Whether these can be obtained by application of further fluorochromes is still under study.
The next aim is to extend it to all soil organisms or groups of organisms to allow complete studies of the soil biotope. Since protozoa could be observed after FDA-fluorochromation already (SAXENA and JENSEN, personal communication) and nematodes partially in the FDA- or in the triple-stained mounts, while bacteria can be stained with fluorochromes like acridine orange, this target seems to be inside easy or at least possible reach (STRUGGER 1949; WYNN-WILLIAMS 1985).
Another aim for further development is the retardation of the photo-fading of FDA. The citifluor antifadant is of some help, but longer lasting observations are still difficult if not impossible. The stable fluorescence of calcofluor white compensates only partial, since the FDA-staining of the metabolically active regions would allow to follow alterations or developments – which are always connected to metabolism – better than with any other substance.
References
- Baath, E. & B. Söderström (1988): FDA-stained fungal mycelium and respiration rate in reinoculated sterilized soil. Soil Biology & Biochemistry 20, 403 – 405.
- Cohen, Y., S. Peter, 0. Balass, & M. D. Goffey (1987): A fluorescent technique for studying growth of Peronospora tabacina of leave surfaces. Phytopathology 77, 201 – 204.
- Correa, B., A. Purchio, C. R. Paula, & W.G. Ambale (1986): Evaluation of a fluorescent method (fluorescein diacetate and ethidium bromide solution) in the study of viability of Cryptococcus neoformans strains. Mycopathologia 96, 91 – 96.
- Dolan, A. & R. J. McNicol (1986): Staining conidia of Botrytis cinerea with calcofluor white PMS and its effects on growth and pathogenicity to raspberry. Transactions of the British mycological Society 87, 316 -320.
- Griffith, 0. H., W. A. Houle, K. F. Kongslie, W. W. Sukow (1984): Photoelectron microscopy and photoelectron quantum yields of the fluorescent dyes fluorescein and rhodamine. Ultramicroscopy 12, 299 – 308.
- Gull, K. & A. P. J. Trinci (1974): Detection of areas of wall differentiation in fungi using fluorescent stain. Archiv für Mikrobiologie 96, 53 – 57.
- Heath, I. B. (1988): Evidence against a direct role for cortical actin arrays in saltatory organelle motility in hyphae of the fungus Saprolegnia ferax. Journal of Cell Science 91, 41 – 47.
- Jensen, C. (1994): Fluoreszenzmikroskopische M6glichkeiten zur in-situ-Beobachtung nematophager Pi1ze im Boden. Dissertation / PhD Thesis, Free University Berlin. Dissertationes Botanicae 217; J. Cramer Verlag, Berlin Stuttgart
- Jensen, C. & G. Lysek (1991): Direct observation of trapping activities of nematode-destroying fungi in the soil using fluorescence microscopy. FEMS Microbiology Ecology 85, 207 – 210.
- Pearce, R. B. (1984): Staining fungal hyphae in wood. Transactions of the British mycological Society 82, 564 -567.
- Raju, N. B. (1982): Easy methods for fluorescent staining of Neurospora nuclei. Neurospora Newsletter 29, 24 – 26.
- Rohringer, R., W. K. Kim, D. J. Samborski, N. K. Howes (1977): Calcofluor: An optical brightener for fluorescence microscopy of fungal plant parasites in leaves. Phytopathology 67, 808 – 810.
- Saxena, G. & G. Lysek (1993): Observation of nematophagous fungi in natural soils by fluorescence microscopy and their correlation with isolation. Mycological Research 97, 1005 – 1011.
- von Sengbusch, P., J. Hechler & U. Müller (1983): Molecular architecture of fungal cell walls – an approach by use of fluorescent markers. European Journal of Cell Biology 30, 305 – 312.
- Söderström, B. E. (1977): Vital staining of fungi in pure cultures and in soil with fluorescein diacetate. Soil Biology and Biochemistry 9, 59 – 63.
- Strugger, S. (1949): In: Fluoreszenzmikroskopie und Mikroskopie. Hannover, Germany.
- Wynn-Williams, D. D. (1985): Photo fading retardant for epifluorescence microscopy in soil microecological studies. Soil Biology and Biochemistry 17, 739 – 346.
